On the afternoon of October 2, Beijing time, the 2023 Nobel Prize in Physiology or Medicine was awarded to Katalin Karikó and Drew Weissman from the United States, for their discoveries in nucleoside base modification, thereby developing an effective COVID-19 mRNA vaccine.

The awards committee said in a press release issued on the same day that the research results of the two winners “fundamentally changed the understanding of how mRNA interacts with the immune system” and are crucial for the development of effective mRNA vaccines during the COVID-19 epidemic. In a time of modern threats to human health, the laureates’ research has made important contributions to the unprecedented speed of vaccine development.
In addition to the application of mRNA drugs in the development of vaccines against challenging infectious diseases (including malaria, HIV and tuberculosis), the industry is also actively exploring mRNA tumor vaccines, and the mRNA track will continue to be hot.
The working principle of the mRNA vaccine is to introduce the mRNA expressing the antigen target into the body through a specific delivery system, label the protein in the body and stimulate the body to produce a specific immunological response, so that the body can obtain immune protection.
Lipid nanoparticles (LNP) are commonly used carriers for mRNA drugs. mRNA vaccines require special modifications or package delivery systems to achieve intracellular expression of mRNA, and delivery technology is a bottleneck limiting the development of mRNA drugs/vaccines. However, LNP is one of the most widely used delivery systems in nucleic acid drug research. The mRNA vaccines currently on the market all use LNP to deliver drugs.
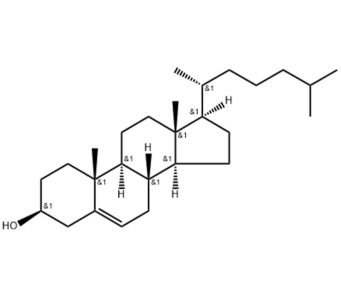
LNP is a multicomponent system usually composed of ionizable lipids or cationic lipid-like compounds, auxiliary lipids, cholesterol, and protective agent polyethylene glycol-lipid conjugates.
Cholesterol is a naturally abundant component of cell membranes and is often used as a structural lipid in LNP formulations. Cholesterol accounts for about 20-50% in LNP formula. Cholesterol is a rich component of animal cell membranes and is usually extracted in large quantities from natural raw materials such as wool. Currently, most of the raw materials for cholesterol products on the market are of animal origin, such as animal brain, spinal cord, tissue, lanolin, etc. Animal-derived products have certain particularities, such as raw materials that are perishable, endogenous residues or exogenous contaminants (such as proteins, microorganisms, viruses, pesticides, veterinary drugs, etc.) that may exist, and compositions and/or composition ratios are unclear. , unique ingredients that are harmful to the human body (such as prion proteins), etc., which may affect the batch-to-batch consistency of the product and even cause unpredictable adverse reactions after use.
But research has found that replacing cholesterol in LNP formulations with natural phytosterols, such as beta-sitosterol and oxidized cholesterol derivatives, can significantly improve mRNA delivery.
QIXIN independently develops, produces and sells cholesterol (plant source), CAS NO. 57-88-5. The product quality meets USP, EP and other pharmacopoeia standards, which can exactly solve the industry’s demand for natural plant sterols to replace cholesterol in LNP formulas. , to achieve better delivery of mRNA vaccines.
Moreover, the plant-origin cholesterol provided by QIXIN New material has been recognized by major pharmaceutical companies around the world because of its following characteristics, and is used in mRNA vaccines and high-end cosmetics.
1. Product origin: Plant-Origin only, from phytosterols(obtained from corn, soybean or pine, etc.)
2. Safety assurance: Non-GMO, TSE/BSE FREE
2. Safe and non-toxic, no animal genetic residues
3. Quality: Conforms to USP, EP pharmacopoeia standards
QIXIN welcomes customers to contact us at any time and look forward to working with you to create a new peak in the industry and contribute to human health and medical care.